Eurus: Simulation Lab testing with ASL 5000 (Image Credit: Christopher Zahner)
Eurus: Patent Diagram (Image Credit: Aisen Caro Chacin)
Eurus (Image Credit: Aisen Caro Chacin)
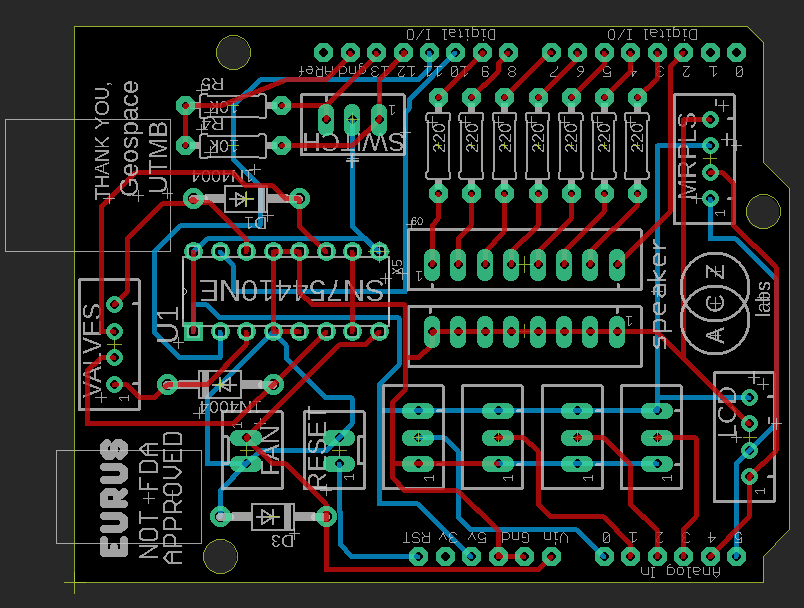
Eurus: Circuit Design (Image Credit: Aisen Caro Chacin)
-
Work Title
エウロス: 血圧カフ緊急蘇生システム
-
Work Title(EN)
Eurus: Pressure-Cuff Actuated Emergency Use Resuscitator System
-
Please describe the concept of your artwork in 2000 words.
Project Summary/Abstract
There is an urgent need for ventilators during the COVID-2019 pandemic. In response to this call for action, we created Eurus, an emergency ventilator prototype that uses readily available medical supplies paired with an electronic module. This design allows for medical staff to supplement care should the need arise if there is a ventilator shortage. The design consists of automating a manual resuscitator (Ambu bag), that is squeezed by a blood pressure cuff which is actuated by the medical air and vacuum ports located in each patient room in the hospital. The air inflates the cuff, squeezing an Ambu bag, and the vacuum quickly releases the air from the cuff, reinflating the resuscitator. This mechanism consists of an electronic unit that uses two electro-mechanical valves and is controlled by 4 dials that set the breaths per minute, approximate tidal volume, Inhalation to Exhalation (I:E) ratio, and inhalation pressure sensitivity. It has a disposable pressure sensor in the patient airway that continuously monitors for safety and to assist patient breaths. The unit is also equipped with an LCD screen that shows the set values and the current patient airway pressure, as well as visual and audible alarms.
The intent of the project is to make a low-cost automation device for manual resuscitators that clinicians can use in the event of conventional ventilator shortage, that provides Control and Assist/Control emergency ventilation and improves the survivability of patients compromised by COVID-19. The focus of the effort includes a scalable design that can be assembled rapidly by medical and clinical staff, that is safe, effective, and reliable.
Patient survival is our first priority, and we assume that this device will be used under strict emergency measures and physician supervision and assessment; risk mitigation precautions are a top priority. Our model takes existing medical equipment to ensure that patient contact with the device is safe and approved by the Food and Drug Administration (FDA). Eurus automates the manual operation of device 21 CFR 868.5905, Resuscitator, Manual, Non-Self-Inflating, product number NHK, class II. In the case of conventional ventilator shortages, manual resuscitators would be the last resort for providing patients with breathing assistance. By automating a manual resuscitator, Eurus alleviates the risk of COVID-19 exposure to medical staff that would otherwise have to manually provide ventilation. This automation also provides more precise ventilation than prolonged manual bagging and reduces the workload on medical staff.
Project Description
(i) Statement of Need
In early March of 2020, the World Health Organization (WHO) declared COVID-19 a pandemic. The disease was first covered in the literature in December of 2019 and in a little over 4 months had spread from one region of China to 114 countries, infecting 118,000 people, and leaving 4,000 dead (1). Governments, hospitals, and the public are looking for ways to slow the spread and diminish the effects of the virus.
Acute Respiratory Distress Syndrome (ARDS) appears to be a major risk factor for death from COVID-19. In one Chinese study, they reviewed 191 patients who developed an infection from COVID-19. Of the 54 patients who died from the disease, 50 of them developed ARDS (2). ARDS is a condition of lung cellular damage characterized by inflammation, edema, and cellular damage. The disease is noted for decreased oxygen diffusion resulting in hypoxemia. Increased oxygen concentration and ventilation with pressure support are effective therapies to treat ARDS.
Given the existing shortage around the world and the potential shortage of ventilators in the coming days or weeks, we have created an emergency ventilator prototype, Eurus. It uses medical supplies from the closets located in each unit of the hospital. This design allows for medical staff to improvise care should the need arise for more ventilators that are available and provides a safer, more reliable alternative than manual resuscitation.
(ii) Overall Goal
The main objective of this project is to increase the number of potential patients who may be ventilated adequately in the event that there are insufficient ventilators to treat patients suffering from ARDS caused by COVID-19.
We need to conduct a bio-compatibility study, to investigate the efficacy, safety, and reliability of Eurus in comparison to manual resuscitator ventilation. This study will allow us to successfully achieve an Emergency Use Authorization (EUA) by the FDA and to manufacture a small number of approved devices ready to be deployed in case of a ventilator shortage due to a resurgence of a high number of COVID cases.
During the development process of Eurus, the FDA and AAMI (American Association for Medical Instrumentation) have put forth new standards and design guidelines specific to this type of device now called Emergency Use Resuscitator Systems (EURS) (4, 5). Because of the fluid nature of these difficult times, navigating regulatory hurdles has been a challenge. We have finally learned the appropriate pathway for approval based on an example submission that has been shared with us by the University of Minnesota. That team successfully received FDA approval through the EUA process for their EURS (6). Their device uses motor-driven linear actuation, rather than semi-radial pneumatic actuation, it has no sensors to monitor the patient, and it only provides controlled ventilation, unable to deliver assisted breaths. We are confident that our design is safer for patients because it provides more sensing and ventilation capabilities. Eurus leaves the Ambu bag completely accessible for clinicians to immediately begin manual bagging in the case of any kind of device failure. Unlike most other EURS designs, the inflatable cuff can easily be squeezed manually while still attached to the resuscitator because it is flexible; it has no barrier to operate the resuscitator as normally directed. Other designs have a mechanical enclosure that prevents the Ambu bag’s standard operation or makes it difficult.
Eurus is easier and cheaper to manufacture because some of the parts are readily available at the hospital and it uses standard fittings and hoses found at local home-improvement stores. The manufacturing consists of a standard waterproof container box that houses the electronic components and valves, requiring no moving parts or specialized vacuum or injection molding.
(iii) Design Specifications
The design of Eurus consists of an inflatable cuff that surrounds a manual resuscitator. The cuff is actuated by the medical air and vacuum ports located in each patient room in the hospital. The air inflates the flexible cuff, squeezing the Ambu bag, and the vacuum quickly releases the air from the cuff, letting the resuscitator re-inflate naturally. This pneumatic actuator is controlled by an electronic module that uses a microcontroller, an H-bridge, and two electro-mechanical valves. The interface consists of 4 dials that adjust the approximate tidal volume, breaths per minute, I:E ratio, and inspiratory pressure sensitivity, as well as an LCD display that shows the current settings and monitors the patient’s airway constant pressure. The device is also equipped with a speaker and LEDs that indicate specific alarms based on ISO 60601 standards (3).
Technical Specifications:
Volumes and breath per minute thresholds: 700mL VT at 30bpm & 450mL VT at 45bpm
FDA-approved patient air pathway pressure sensor, approved for operation in high oxygen environments
Mechanical control pop-off valves of 40- 60cmH₂O
Mechanical PEEP valve of 5-20cmH₂O
I:E ratio, tidal volume, breaths per minute, and inspiratory sensitivity are controlled by adjustment dials
Watertight container and it fits in a 16”x16”x16” box, rated IP22
Uses ¼” standard hoses and fittings found in supply stores
Device supply: 12v 1.5amps, Class II, Continuous operation.
Audible and visual alarm, power-loss switching module.
LCD displaying dial values and patient airway pressure.
Uses ambient air, Luer lock for O2 injection at the inlet of Ambu bag.
The Future of Eurus
We aim to expand the use of Eurus beyond a mitigation strategy for conventional ventilator shortage during the COVID-19 pandemic. The expedited approval mechanism will afford us the ability to test and deploy rapidly and better understand other needs that can be fulfilled by this product in the eventuality of commercialization. This includes the ability to equip ambulances with automated resuscitators and free the hands of first responders when needing to ventilate a patient en-route; therefore, providing better care for patients and increasing their chance of survival. Another scenario where Eurus can be useful is on ad-hoc hospital tents in remote areas or battlefields. The portability, lightness, and low power requirements of the device can afford clinicians in these circumstances better functionality and a greater number of ventilators to be available for care. In the case of remote operation, we have already tested the functionality of the device while operating with a garage compressor and a shop-vac to supply the air that inflates the cuff. This system for actuation can operate various Eurus devices simultaneously.
Beyond the commercialization of Eurus, we expect that we can significantly contribute to medical device prototyping and approval strategies in academic journals. We also hope that this device can serve as an example of the innovation and rapid development capabilities that represent UTMB, the Galveston community, and Houston's extended community as a whole. Our unique position to prototype, test, and develop a device that serves to prepare our community in such a time of need is exemplary for the impact that our organization can have, offering safe, reliable, and quick solutions, developing and fostering community trust, and most importantly ensuring that patient care is our first priority.
Community Support
COVID-19 has created a circumstance for community solidarity and willingness to cooperate in ways that are unprecedented. Our project has received support from many individuals and organizations across Galveston, the Houston area, the state, and even internationally. Companies and institutions such as Accenture, GeoSpace Technologies, Airgas, Texas Tech, Texas A & M, University of Houston, NIST, and NASA have shared their resources, time, and effort in kind to contribute to the project. GeoSpace Technologies is willing to forego their profit margins to produce the devices at cost, and Dr. Perenlei Enkhbaatar MD, Ph.D., FAHA, Pr. Anesthesiology, and Director of Translational ICU has offered to help us to circumvent some animal testing costs by providing some of their services free of charge. We are eager to continue our efforts and to make sure that we have a plan in the event of a ventilator shortage, and a way to contribute to other medical facilities in areas that may be less fortunate. We know that this initiative will not only have the potential to save lives in our community but also inspire technological innovation that is accessible and rapidly deployable. We are so proud to have received so much support, and we are honored to serve with our creativity to better prepare our community.
References:
1. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment Coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published online ahead of print, 2020 Mar 11] [published correction appears in Lancet. 2020 Mar 12;:]. Lancet. 2020; S0140-6736(20)30566-3. doi:10.1016/S0140-6736(20)30566-3
3. ISO, “IEC 60601-1-8:2006 Medical electrical equipment — Part 1-8: General requirements for basic safety and essential performance — Collateral standard: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems.” https://www.iso.org/standard/41986.html
4. FDA, “Appendix B: Authorized Ventilators, Ventilator Tubing Connectors, and Ventilator Accessories.” Updated: May 18, 2020 https://www.fda.gov/media/136528/download
5. AAMI, “Consensus Report, Emergency Use Resuscitator Systems Design Guidance.” AAMI/CR503:2020https://www.aami.org/docs/default-source/standardslibrary/200410_cr503-2020_rev1.pdf
6. Hinton, Denise M. “Emergency Use Authorization (EUA) issued in response to concerns relating to insufficient supply and availability of FDA-cleared ventilators for use in healthcare settings to treat patients during the Coronavirus Disease 2019 (COVID-19)1 pandemic”. FDA, https://www.fda.gov/media/136423/download -
Please describe the concept of your artwork in 2000 words. (EN)
-
Work Specification
Dimensions: 70cm x 40cm x 20cm
Materials: Silicone, PET plastic, Brass, Nylon, Stainless Steel, Electronics
Specifications:
800mL VT at 30 bpm & 450mL VT at 45 bpm
FDA approved patient air pathway pressure sensing
Mechanical control pop-off valves of 40- 60cmH₂O
PEEP at 5-20cmH₂O (Ambu bag parts.)
Uses ambient air, luer lock for O2 injection at inlet
¼” standard hoses and fittings found in supply stores
Watertight container and it fits in a 16”x16”x16” box
Device power supply: 12v 1.5 amp (air & vac need generators if operating remotely)
PVC valves stress-tested 62 hrs+
Audio + visual alarms
Microcontroller: ATMega328PU
Controls: Breath sensitivity, Breaths per minute, Volume, and I:E ratio
Medical grade pressure sensor
Control modes: Time and Assist/Control
Eurus Exhibition Diagram: https://docs.google.com/presentation/d/19pmubuscNSYDyLXNav5esA4pt7nM1BW8WGWIXPUvwWU/edit#slide=id.ge5dfcdf44d_0_2 -
Work Specification(EN)
-
Media CoverageURL
http://artsci.ucla.edu/node/1470 https://ars.electronica.art/aeblog/en/2021/08/21/the-future-of-work/ https://www.nytimes.com/2020/03/30/health/coronavirus-innovators.html https://abc13.com/coronavirus-device-doctor-creates-houston-doctors/6046547/ https://ars.electronica.art/aeblog/en/2020/04/22/ki-corona-part2/ https://www.galvnews.com/news/free/article_dd33235e-3336-5e2a-9e6e-dd28b0189c49.html
-
Video URL
https://youtu.be/TnQzDPvjvRg
-
Your OfficialURL (Website, Instagram, Facebook)
https://aczlabs.com/ and http://aisencaro.com, @aisencc
-
Please describe how your work relates to the theme of the special prize.
Panasonic Special Prize
Eurus represents an incessant drive to survive, no matter how difficult the challenge. This project was born from the will to live and save lives, with the impetus that we can find solutions with what we already have at hand, if only we allow ourselves to look at our resources in a creative and inventive way.
In March 2020, ventilators were the only answer to COVID-19. At the time, there was no cure, no vaccine, no known treatment, except to help our patients breathe while they battle the virus. We saw Italian doctors on the news making the impossible decision of choosing who would get a ventilator and who would be left at the mercy of their immunity. It was at this very moment that we realized we had to do something. So, we set out to design a low-cost, replicable, accessible, safe, and simple device. Our main goal was to create a ventilator with as many hospital supplies as possible and very common electronic devices. This way we could publish the plans and every hospital could gather a team of nearby engineers to help them assemble the device.
The ventilator impulse was a phenomenon that surged massively, collectively, and globally. A call to action to make ventilators in the open-source community began to brew. It was a push for the need to survive, to pull together our resources, come up with our best solution, and share it with the world in hopes that someone else improves on it and makes it work for the collective well-being.
Our project was born out of a single circumstance that crippled all of society at once, a time where we sought to survive, share, and cooperate for the chance to all thrive together. These difficult times made us look back and learn from the outcomes in the past and to see what our ancestors have done to thrive in times of hardship. “If we as humankind are destined to be in that situation and can't escape from it, then perhaps it can't be helped. [...] I thought otherwise: Humans are naturally endowed with the possibility for limitless prosperity, peace, and happiness.” -Konosuke Matsushita, 22nd anniversary of PHP Institute, Inc. on November 3, 1968.
This need to live for the possibilities and to believe by doing, in overcoming the obstacles to achieve an ideal life, is the same philosophy that drove us to build Eurus.
Eurus did not only serve as a possible solution for the global ventilator shortage, it was a source of energy, belief, hope, and pride that helped us, our hospital staff, and our community to continue putting forth their strength for the pandemic’s challenges. Our project is made in the same spirit as Mr. Matsuhita’s waterworks philosophy: to energize society, and improve life by making devices accessible and available. Eurus is an inspiration to clinicians to know that they are not alone with the burden of treating patients, an inspiration to society to know that we are innovating and sharing all of our knowledge and resources to come up with solutions, and the support that we received from our community is an inspiration for us to continue building technology that is resourceful and accessible to all.
In a world where manufacturing is a well-oiled machine, that moves seamlessly from idea to consumer, we had to reinvent how to massively scale an idea where the usual processes were completely shut down. During this project, being resourceful meant local expertise and sparse production; our approach was to imagine there was no shipping, no availability of parts beyond what we had at the time on the ground. We learned that the only way to persevere through the pandemic was by having an energetic, symbiotic, and dynamic approach. Social resilience is inherent in Eurus in every aspect of this democratic experiment, an idea to remain alive and to band together to subsist with the force of collaborative and creative innovation.
It is an honor to present Eurus as a project that aligns with the same values that established Panasonic, a company founded by such an incredible man full of ingenuity and generosity to improve life and make technology accessible to all.